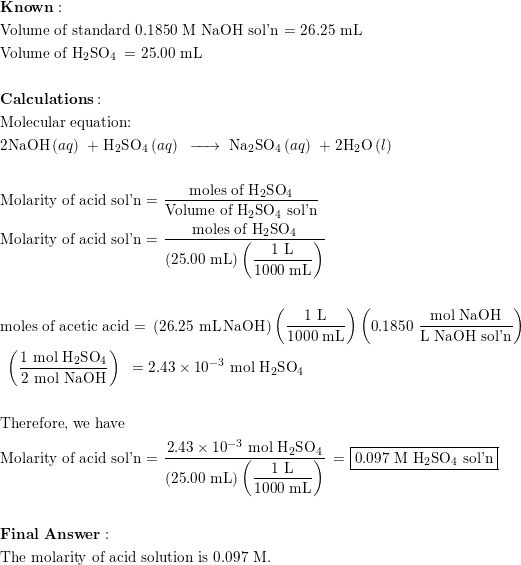
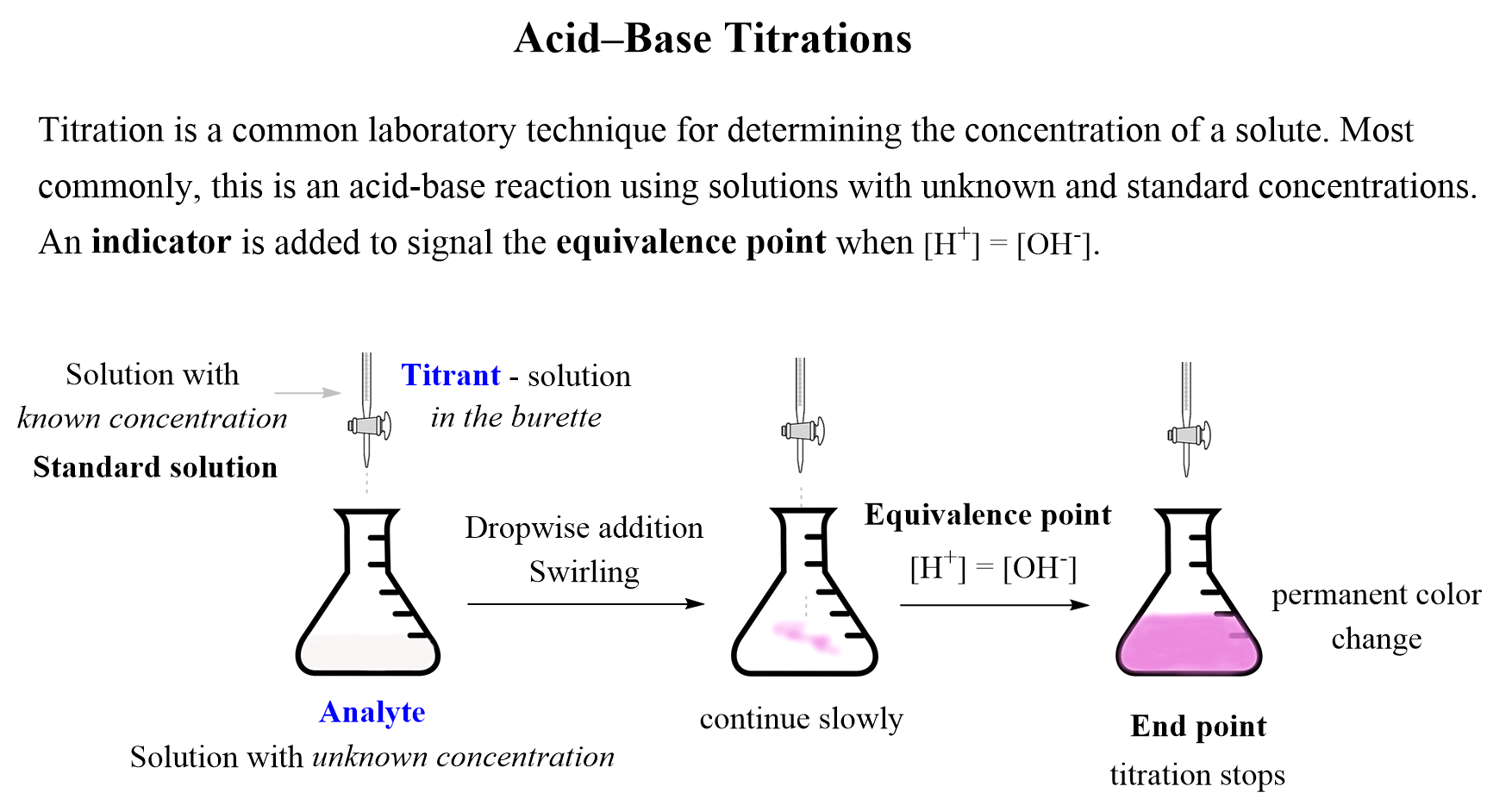
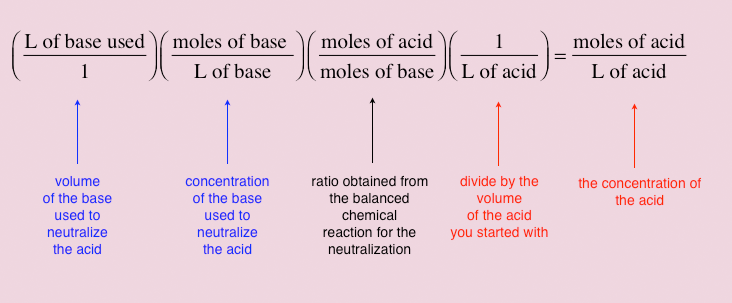
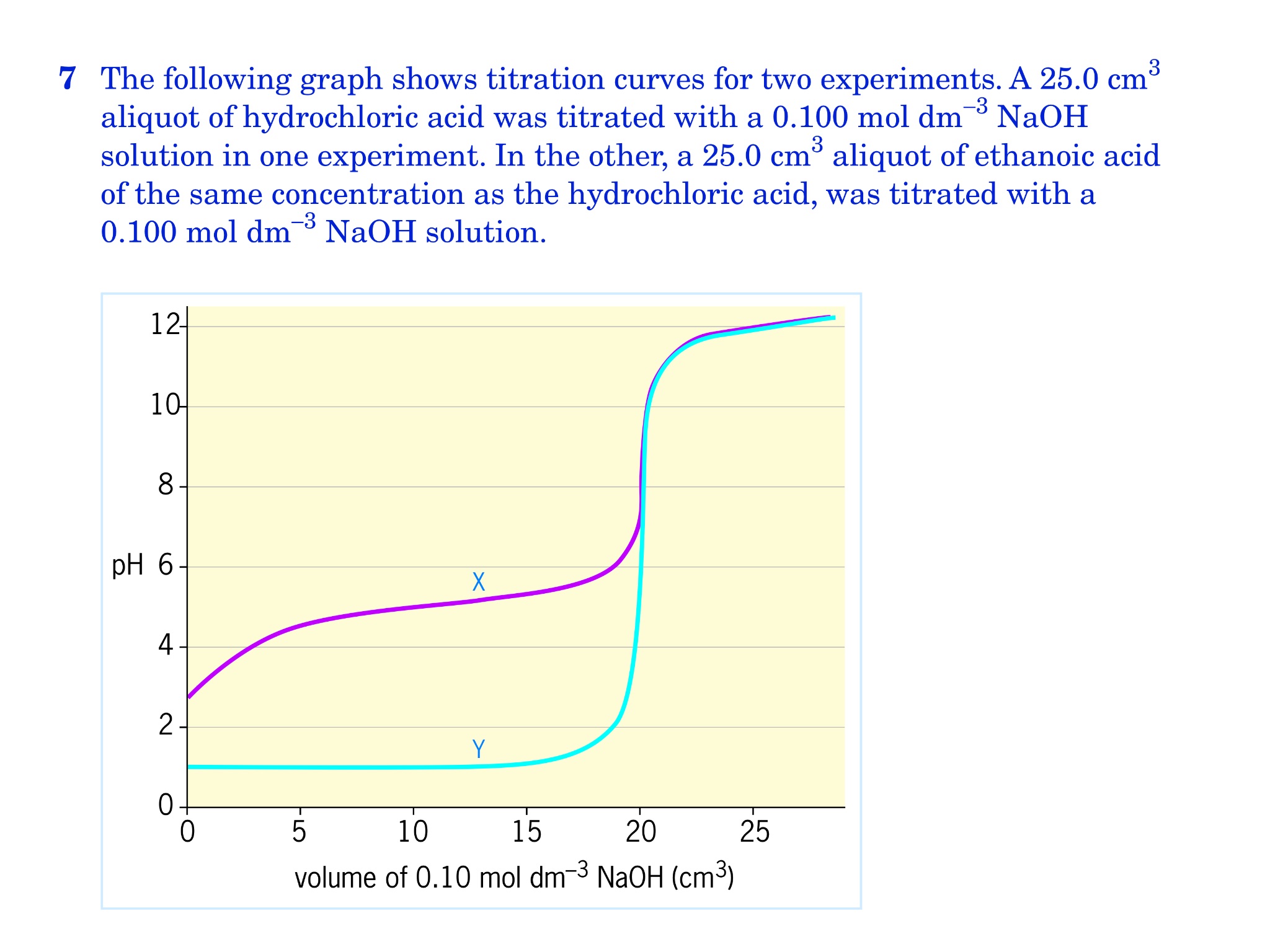
![Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download](https://slideplayer.com/10846268/39/images/slide_1.jpg)
Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download
What volume of 0.1mol/dm3 hydrochloric acid will be required to neutralize 20cm3 of 2.0mol/DM3 sodium hydroxide? - Quora
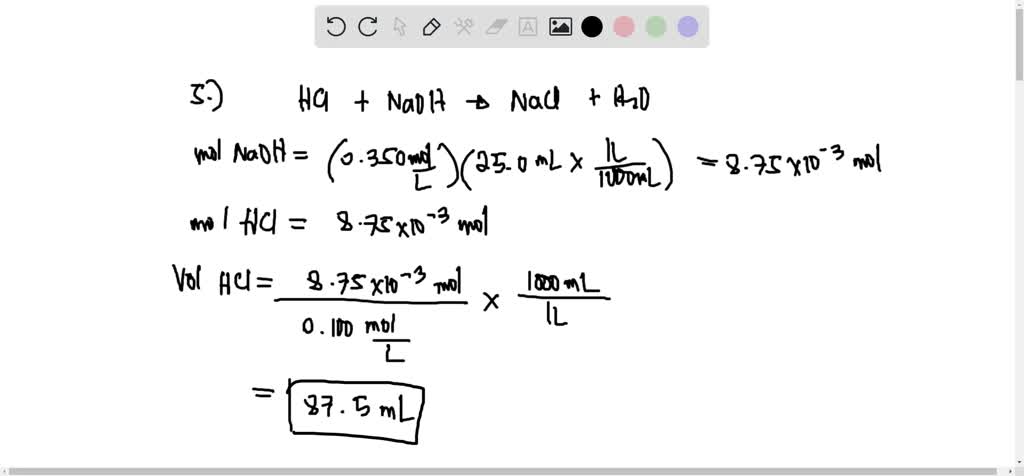
SOLVED: 5. Calculate the volume of 0.100 M HCI solution needed to neutralize 25.0 mL of 0.350 M NaOH solution. (Answer: 87.5 mL) Calculate the volume of 0.100 M HzSO4 solution needed

Neutralizing Solutions with Sodium Hydroxide | Process & Chemical Formula - Video & Lesson Transcript | Study.com
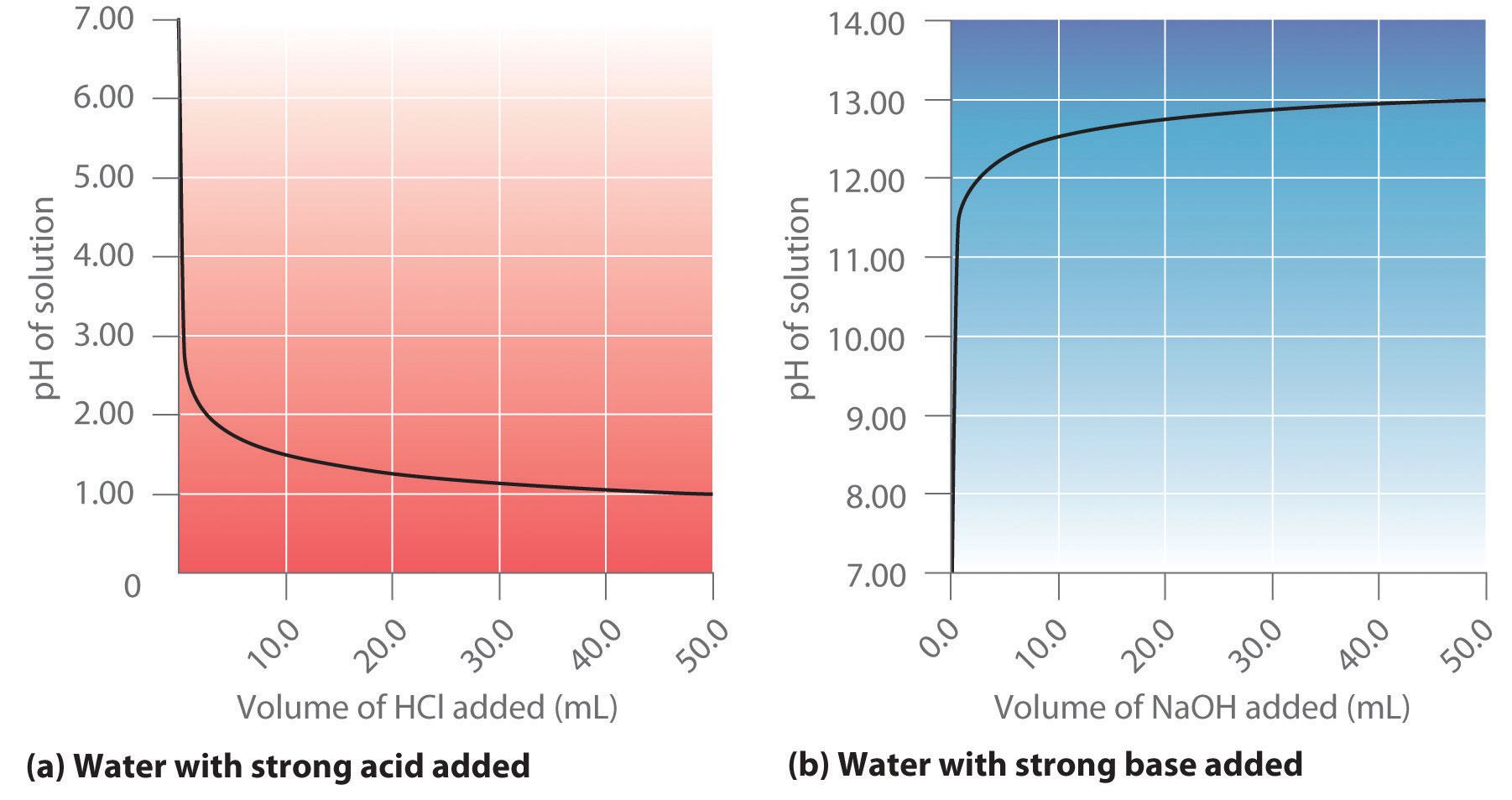
![Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download Tuesday May 26 Objective: Calculate the amount of acid or base needed to neutralize a solution. Checkpoint: – Calculate the [OH-] in a solution that has. - ppt download](https://images.slideplayer.com/39/10846268/slides/slide_7.jpg)